Even as many Americans line up for COVID-19 vaccines, others – including those who have previously expressed trust in the medical establishment and vaccines – have conveyed strong reservations. The reasons behind this hesitancy differ, but core concerns focus on the speed of development and its effect on vaccine quality and safety.
Understanding the Vaccine Development Process
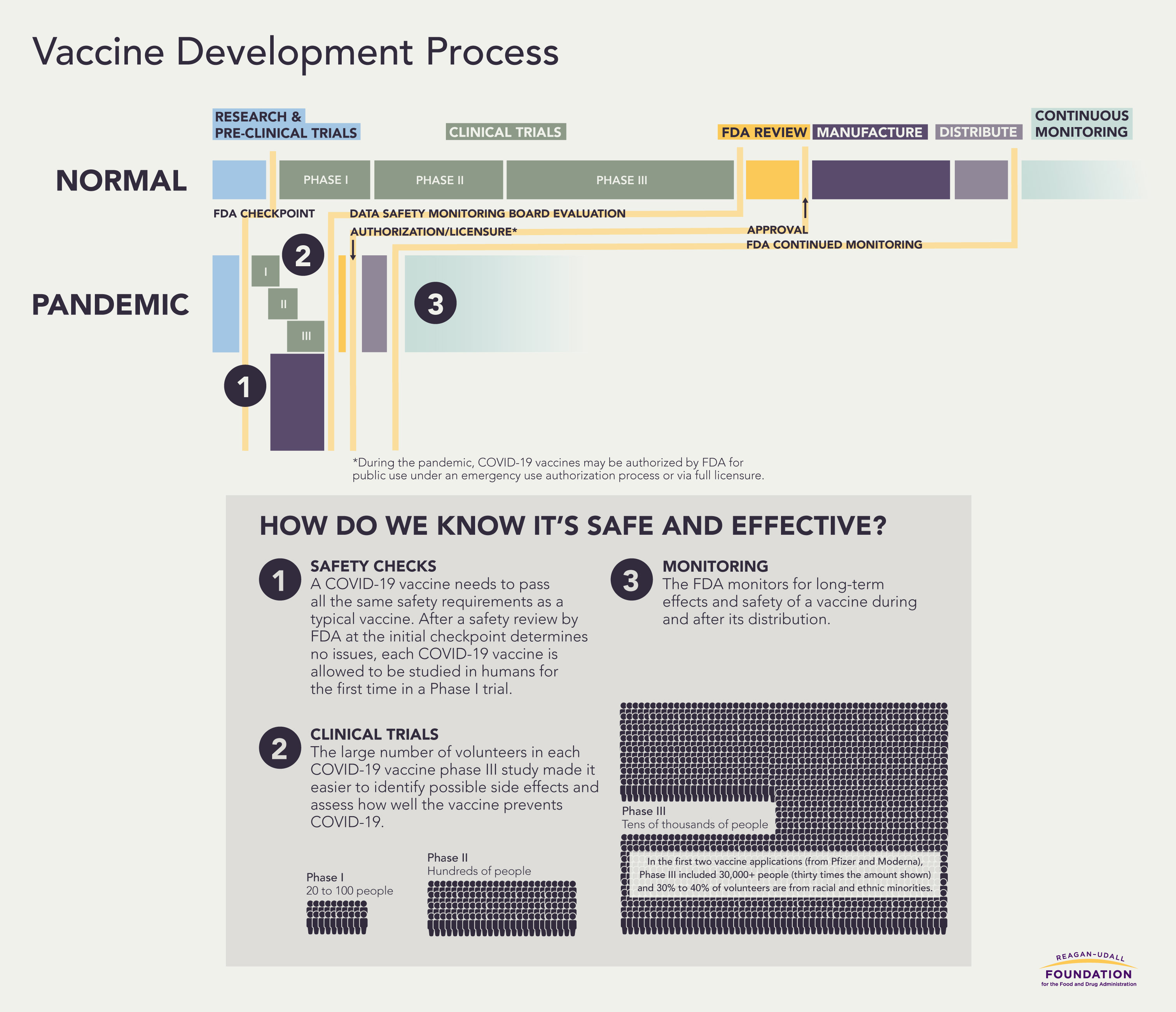
How do we know it’s safe and effective?
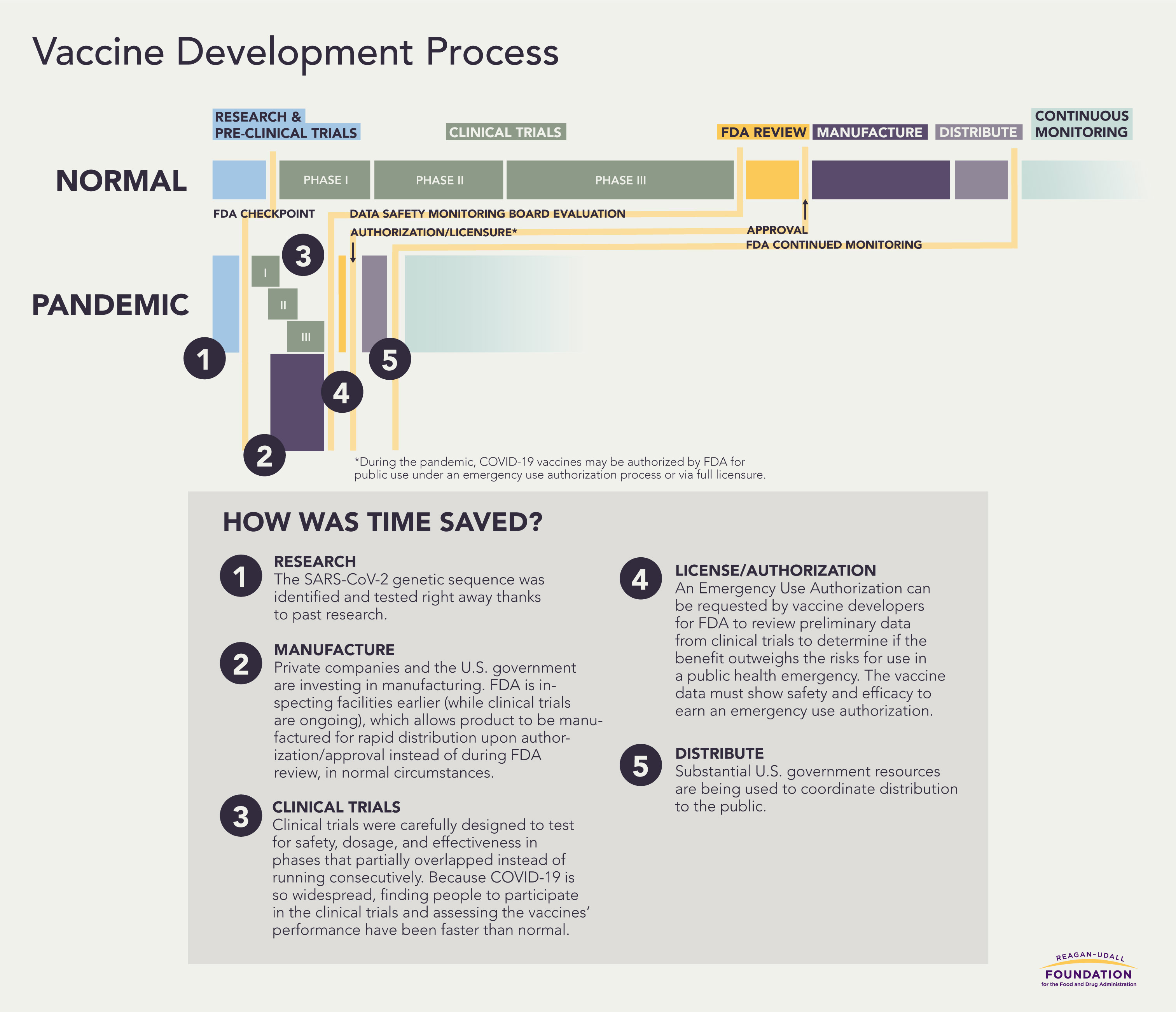
How was time saved?
The Reagan-Udall Foundation for the FDA worked closely with the U.S. Food and Drug Administration’s Center for Biologics Evaluation and Research to better understand the perceptions that lead to COVID-19 vaccine hesitancy and to identify credible messages and messengers. Focused specifically on communication about FDA’s role in vaccine review and authorization/approval, the Vaccine Confidence Project aims to provide the vaccine-hesitant with the information they need to decide whether or not to receive a COVID-19 vaccine. View the Executive Summary Project Slide Deck.
The Vaccine Confidence Project began with listening. Our focus was to hear from front line workers and traditionally under-represented populations. We shared our then-work-in-progress with FDA’s Vaccines and Related Biological Products Advisory Committee in October 2020. You can watch the full Advisory Committee meeting here (Vaccine Confidence Project findings are at the 3:35:30 mark) and view our slide deck.